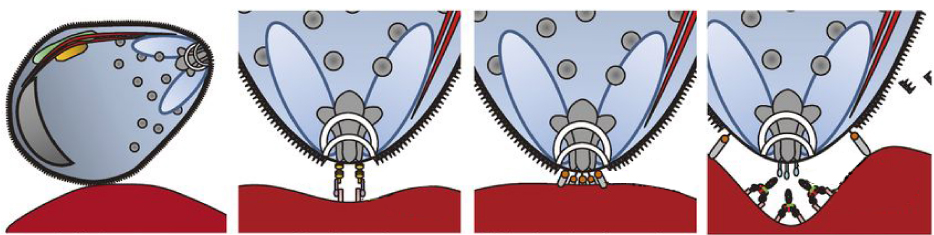
| Malaria | We pay special attention to drug development for the third world, as an integral part of our global health care strategy. In Africa 180 million people are suffering from Malaria. In 2018 alone,among the 445,000 died of malaria, 92% died in Africa. One of the major infections in tropical countries is from the bite of the female mosquito Anopheles, which infect people with the Plasmodium parasite. There are five types of parasites: Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium tipterygium and Plasmodium knowlesi. Among them Plasmodium falciparum was reported to have the highest incidences, short course, and highest mortality. The rhoptry-associated protein 2 (RAP2) interacts with CD147 on the surface of erythrocytes. This CD147 membrane protein has been the focus of our basic research for more than 30 years. With this expertise, we developed a humanized monoclonal antibody, Meplazumab which blocks CD147, the receptor for Plasmodium falciparum, preventing penetration of parasite into erythrocytes and thereby inhibits the progression of malaria infection.
Presently an IND is approved by US FDA with orphan drug designation and Fast Track designation, with phase I clinical trials undergoing in Australia.
Meplazumab is expected to become world’s first antibody therapeutics to prevent and treat malaria, solve the drug resistance problem and provide effective treatment for malaria patients. It is projected to bringing gospel to millions of people in Africa and around countries the world.
Presently an IND is approved by US FDA with orphan drug designation and Fast Track designation, with Phase I clinical trials undergoing in Australia. |